Dr. Kara Fitzgerald details her publication by Aging, entitled, “Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial“.

Behind the Study is a series of transcribed videos from researchers elaborating on their recent oncology-focused studies published by Aging. A new Behind the Study is released each Monday. Visit the Aging YouTube channel for more insights from outstanding authors.
—
Hi, I am Kara Fitzgerald. I’m on faculty at The Institute for Functional Medicine. I have a clinic practice in Newtown, Connecticut. The title of our paper is “Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial“.
We became interested in epigenetics because we practice functional medicine. So we’re concerned with genetic expression. Particularly, I would say that my first big wake-up call came from the research in cancer, epigenetics where the tumor micro environment hijacks epigenetic expression kind of takes over hypermethylating tumor suppressor genes, turning on oncogenes, et cetera. Our question became if we are pushing methylation forward with high dose methyl donors such as full later B12, could we be influencing cancer, epigenetics at all? That and a few other reasons prompted us to develop the diet and lifestyle intervention and try a very nutrition forward approach to changing epigenetic expression.
However, we can’t get an Illumina EPIC array in clinical practice. And so once we designed the program and started to use it in clinical practice, the next question was: Are we making a difference at all, in epigenetic expression? We were given an unrestricted grant by Metagenics. Metagenics is a professional supplement company out of California so they were not involved in study design. They had no control over the study and/or findings or investment in products that we used.
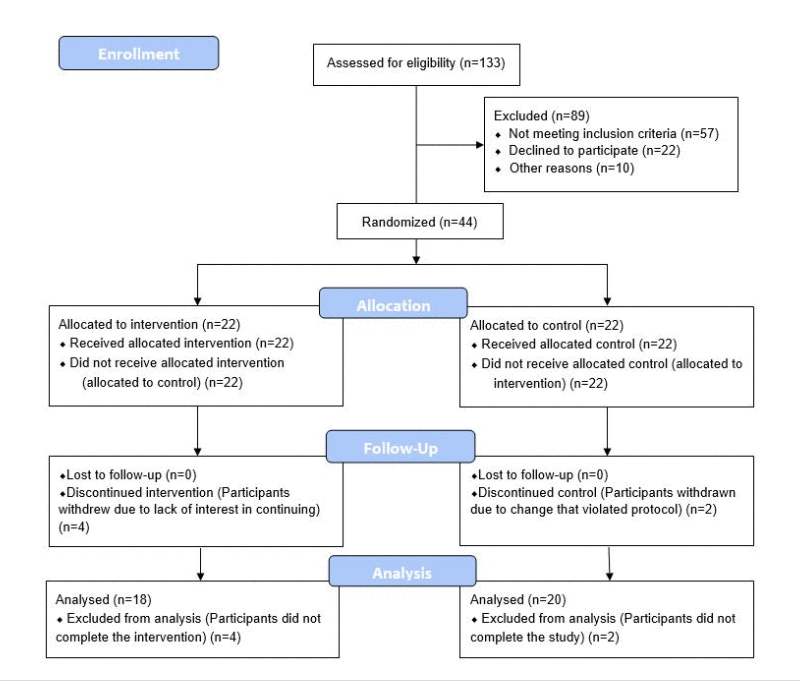
We hired Helfgott Research Institute out of National University of Natural Medicine to run our study. So I, myself and my colleague Romilly Hodges who designed the program, we were not involved in the execution of the program. So that’s a little bit of the background. And what we did was we had a pilot study. We looked at men between the ages of 50 and 72.
We didn’t include women, because at that age range … so, we wanted to look at middle-age when we know DNA methylation starts to go awry, global hypomethylation with those regions of aberrant hypermethylation … so that was the time we wanted to look at, but we didn’t have enough money to have a larger population. So if we included women in that age range, we would have premenopausal perimenopause and post-menopausal subjects, and it would be difficult for us to tease out that influence on the findings. So we decided in our pilot to just go with men and we did our eight-week diet and lifestyle intervention. The diet is again, designed specifically to influence methylation. It’s very methyl, donor dense. There are a lot of greens. There are other nutrients that can influence the methylation cycle, such as beets, choline from eggs.
We encouraged people to have liver a few times a week, which is high again in folate and B12. We also included a lot of the polyphenols that have preclinical data on them for influencing DNMT and Tet enzymes. In fact, a lot of really interesting research, again, going back to cancer, epigenetics, and these polyphenols actually influencing the re-expression of hypermethylated and inhibited tumor suppressor genes. So we were interested in that particularly because a lot of those polyphenols actually have very long traditional use history. So for instance, curcumin or EGCG or resveratrol, luteolin, lutein, ellagic acid, quercetin. When you look into traditional medicine, we see of course millennia long use for green tea and curcumin by way of example, but they’re all pleiotropic in their effect: Anti-inflammatory, antioxidant, anti-tumor agenetic, et cetera. And at least some of those mechanisms I suspect are driven by epigenetic changes.
So diet heavy methyl donors, but also these methylation augmenting polyphenols. We included an exercise prescription, which was at least five days for 30 minutes at a perceived exertion of 60 to 80%. So not necessarily intense. We tracked sleep and encouraged them to get at least seven hours per night and gave them some basic sleep hygiene tips as they requested. There was a meditation intervention as well. So everything that we did has some evidence in the literature, either in clinical studies or preclinical of influencing favorably DNA methylation. We use two supplements, a prebiotic lactobacillus plantarum. We did that specifically because there’s some evidence that lactobacillus plantarium may increase that endogenous microbial production of folate, of natural folates. And we also included a greens powder. So again, the polyphenols that I just mentioned, those in a concentrated powder and our participants took each of those supplements twice a day.
Outcome, we looked at the EPIC Illumina array. We looked at a host of blood biomarkers, subjective questionnaires. Our chief finding, our most exciting finding, was using Horvath … we collected saliva and then using Horvath’s 2013 DNA methylation biological clock we showed a significant reversal of biological age in our subjects by 3.23 years as compared to the control group and that was a P value of 0.018. The within group change in our study participants was 1.96 years, so almost 2 years with a trend towards significance. The P value there is 0.066, so super excited about that finding. We’ve got more to unpack on the Illumina array. Triglycerides dropped in our study participants and LDL dropped in the study participants. Now I should state, I didn’t mention at the beginning, but these were healthy men, not on medication. We had a pretty strict criteria for enrollment.
It actually took us a while. We started this study in 2017. It took us quite a while to enroll because the program was rigorous and the selection process was relatively involved. Circulating folate, circulating methylfolate increased also in our study participants. I think that covers most of it.
We worked with nutritionists. This is another good point. Again, the program is rigorous and we had nutritionists support the study participants. They didn’t do any coaching. They actually just had an IRB approved script where they asked them if they had questions on the diet and then questions on exercise, et cetera, et cetera. So they were required to have some contact with the nutritionists. We had high adherence findings, and I look forward to publishing those and just exploring it. Nutrition interventions are notoriously poor, and I think we actually did well. I suspect it’s because we had these nutrition contact points with the subjects. To my knowledge, it’s the first of its kind study, randomized control study.
It was a double blind obviously, but it was a randomized control study where we had 20 in the control group and 18 in the study group, what else? It was eight weeks in duration. The other diet intervention, as we wrote about in the paper is the new age study and that was a Mediterranean diet over the course of the year. And they had some interesting epigenetic DNA methylation changes and a subgroup of that population did have lowering of biological age.
I want to thank Metagenics for their grant. I want to thank our team. Again, we worked with Helfgott Research Institute, National University of Natural Medicine in Portland, Oregon. My Co-PI from Helfgott is Ryan Bradley, statistician from Helfgott is Douglas Hanes. Emily Stack was the study manager. My team included Romilly Hodges, who is the nutrition director here at our clinic. She helped design. She and I designed the program. The other nutritionists involved are Janine Henkel, Melissa Twedt, Despina Giannopoulou, Josette Herdell and Sally Logan. At McGill are Dr. Moshe Szyf and David Cheishvili, both helped with data analysis, particularly of the Illumina EPIC array. And Dr. Szyf also helped with study design.
So a big team, thank you to Dr. Steve Horvath and Dr. Josh Mitteldorf. Josh worked on Horvath, the DNA methylation clock analysis with some guidance from Steve Horvath. And so we’re deeply appreciative that work for us.
That’s our study. Our future is what we want to continue to look at this. I mean, this was our pilot study and we’d like to do a longer study, a larger study with men and women. So stay tuned, thank you.
Click here to read the full study published by Aging.
Click the links below for more information on corresponding author, Dr. Kara Fitzgerald:
Biological Aging Summary | Instagram | Facebook | Twitter | General Site | Younger You Program
—
Aging is an open-access journal that publishes research papers monthly in all fields of aging research and other topics. These papers are available to read at no cost to readers on Aging-us.com. Open-access journals offer information that has the potential to benefit our societies from the inside out and may be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact [email protected].








